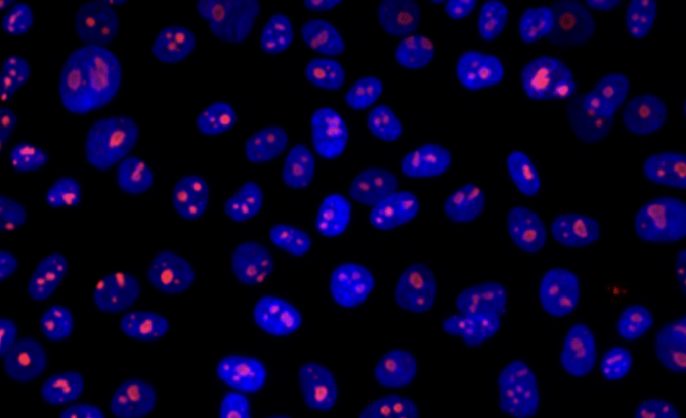
Yale Flow Cytometry Facility
About the core
The Yale Flow Cytometry Facility offers a comprehensive range of services for flow cytometric analysis and sorting, encompassing instrumentation, technical assistance, training, and consultation throughout Yale School of Medicine. Flow cytometry is a technology that simultaneously measures and then analyzes multiple physical characteristics of single particles, usually cells, as they flow in a fluid stream through a beam of light. The properties measured include a particle’s relative size, relative granularity or internal complexity, and relative fluorescence intensity. These characteristics are determined using an optical-to-electronic coupling system that records how the cell or particle scatters incident laser light and emits fluorescence. Any suspended particle or cell from 0.5–150 micrometers in size is suitable for analysis. Cells from solid tissue must be disaggregated before analysis. Sorting allows us to capture and collect cells of interest for further analysis. Once collected, the cells can be analyzed microscopically, biochemically, or functionally.
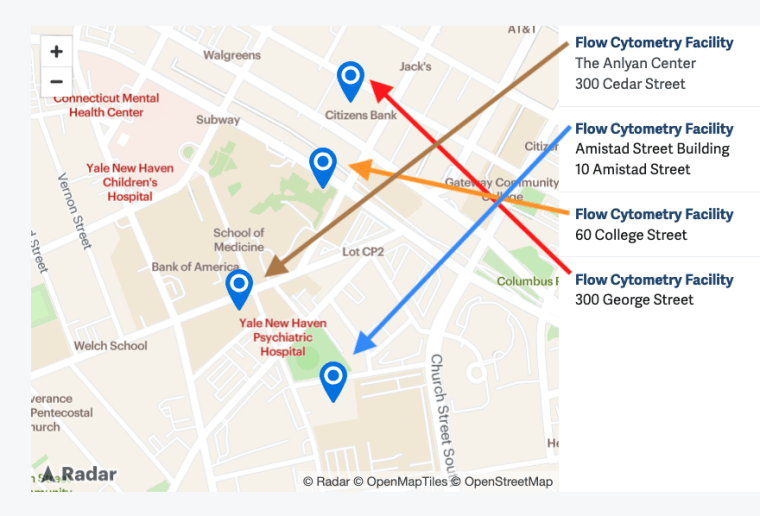
Locations
- The Anlyan Center (TAC) - 300 Cedar Street
- 300 George Street
- Amistad Street Building - 10 Amistad Street
- LEPH - 60 College Street
Publication acknowledgment
If research supported by this research core results in publication, please acknowledge this support by including the following in your publication(s): "We thank Yale Flow Cytometry for their assistance with _________ service. The Core is supported in part by an NCI Cancer Center Support Grant # NIH P30 CA016359. The BD Symphony was funded by shared instrument grant # NIH S10 OD026996. The Sony MA900 cell sorter within the BSL3 facility was funded by shared instrument grant # NIH S10OD036350.”
Available to Yale researchers & external researchers
Onboarding and scheduling
Do you need flow cytometry analysis, cell sorting, or both?
- Analysis results in data recorded by acquisition software.
- Cell sorting gives you cells separated into pure populations and all the data acquired during sorting.
All scheduling, training requests, and services are made through an online portal, PPMS. Your Yale NetID and password will serve as your account credentials. (Download a short guide to this intuitive login.) Please confirm your lab affiliation (“group”) and charging instructions. We also recommend that you check that your permissions for instrument access are correct. In most cases, details about your account may pre-populate, but new users and those without affiliated COAs will need to wait for the business office to approve the COAs you list before your account is functional. There might therefore be a short delay after your account creation before you can book equipment with this system. Once your account is functional, you will be able to book instruments and review reservations with a much more sophisticated interface.
PPMS Quick Start Manual
PPMS Reservation System for Analyzers
PPMS Guide for Booking Cell Sorters
Training Request Guide
Schedule for analysis
The Yale Flow Cytometry Facility is located on the 5th and 6th floors of TAC, in the Amistad Building, and 300 George Street. The flow cytometry analyzers may be independently operated once users have undergone training. Training sessions can be requested in PPMS and will be coordinated through core facility staff.
To schedule, log onto the PPMS scheduling online portal with your Yale NetID and password. (Download a short guide to this intuitive login.)
- Please confirm your lab affiliation (“group”) and charging instructions. We also recommend that you check that your permissions for instrument access are correct.
- In most cases, details about your account may pre-populate, but new users and those without affiliated COAs will need to wait for the business office to approve the COAs you list before your account is functional. There may be a short delay after your account creation before you can book equipment with this system.
Once your account is active, you will be able to book instruments and review reservations with a sophisticated interface.
- A single person cannot sign up for more than 8 hours per week of "prime time" on the cytometers (i.e. no more than 8 hours total). Prime time is Monday-Friday 12N-6PM. An unlimited amount of time may be reserved out of "prime time." No more than 4 hours of "prime time" should be reserved in any single block. If more than 4 hours of time is needed, individuals are encouraged to sign up so as to start before "prime time" or end after it, or use the weekend time. Please contact Diane Trotta if your experiment requires more than the allotted four hours of prime time per day for special permission.
- Users will be charged for the greater of time scheduled or actual time used on the cytometers.
- There is 24/7 access (no key required) to the analyzers in TAC and in Amistad Room 105. Card reader access is required for 300 George St Lab 2331.
Scheduling a cell sorting experiment
Cell sorting is performed mostly by facility staff. First-time sort users are required to fill out a biosafety questionnaire along with the experimental information in their PPMS sort request to ensure the experiment is compatible with the selected sorter. This includes experimental design, e.g. the fluorochromes being used, diagrams of the cell populations, amount of sorted material expected, and sorting regions desired. Sorting availability and scheduling can be booked using PPMS and may require VPN connection for off-campus access.
Users are able to cancel their sort time online. In case of delay or cancellation, notify cell sorter facility personnel as soon as possible by phone or e-mail. Users who cancel their sort less than 24 hours prior to the scheduled time will be charged for their entire scheduled sort time.
| Location | Phone | |
|---|---|---|
| TAC Aria-A (S617) | 203-737-2891 | zhao.zhao@yale.edu |
| Aria at Amistad | 203-785-2299 | ewa.menet@yale.edu |
| Aria at 300 George Street | 203-737-7452 | r.duggan@yale.edu |
| Sony at LEPH | 203-737-5959 | lesley.devine@yale.edu |
| Sony SH800 | 203-785-7958 | zhao.zhao@yale.edu |
| Bigfoot Left & Right | 203-785-7958 |
- Individuals who cancel sorts with < 24 hrs notice on two occasions will not be able to reserve or use the machine for two weeks and if they repeat after this, they will forfeit the right to reserve or use the sorters.
- Anyone can fill cancellations at any time without these restrictions being in effect.
- If the above prevents you from doing an experiment and you need an exception, please contact Flow Cytometry Facility Director Dr. Ann Haberman.
Independent cell sorting
Users with advanced flow cytometry backgrounds may be trained to conduct their own independent sorting experiments on the SONY SH800. For additional information and to schedule a training session please contact Zhao Zhao.
Resources
- FlowJo site license for Yale
- Hallway workstation for Diva exporting
- Remote data access
- Helpful links
- FlowJo tutorials
- Aurora Tutorials
- Flow Cytometry Sorting Tips
Policies & protocols
Policies
Flow cytometry: rules for analyzers
Flow cytometry: rules for cell sorting
Flow Cytometry Facility biosafety
Analysis rooms TAC S613, S617, S533, 300 George Street Rm 2331, Amistad Room 105
- All infectious, human, and non-human primate cells must be fixed before they are analyzed on the cytometers.
- Gloves should be worn by all users at all times.
- No food or drink is permitted.
- Change in the cleanup procedure: The standard 5 minutes of bleach and 5 minutes of dH20 cleaning is only required if you are using infectious, human, or non-human primate samples.
Protocols
Staining recommendations
The basics of staining for cell surface proteins, including direct and indirect staining, solutions, antibody titrations, etc. are well described in the literature. Below are some important recommendations for choosing fluorochromes.
- The fluorochromes must be able to absorb light from the lasers present and must emit at different wavelengths from each other so that their fluorescence signals can be distinguished. The farther apart their fluorescence peaks, the easier it will be to resolve their signals without recourse to excessive compensation.
- The brightest fluorochrome should be assigned to the antibody binding to the protein with the lowest expression. Generally, PE and PE-based energy transfer conjugates have the brightest fluorescence. In the case of cells with high autofluorescence, allophycocyanin (APC) yields more relative brightness than PEs. The combination of PerCP and PE as labels is an ideal choice for measuring a low-density antigen (PE label) on a subpopulation defined by another antigen of medium or high density (PerCP label). There is no significant spectral compensation needed between PerCP and PE. If expression is very low, it may, however, be necessary to test antibodies conjugated to a range of different fluorochromes as conjugation procedures are variable, and it is not always possible to predict brightness from photochemicals principle alone.
- Acidic buffer conditions should be avoided during the analysis of samples stained with FITC because the fluorescence of the dye and its conjugates is pH dependent.
- Most fluorescent labels are light-sensitive. To prevent artifacts, samples should be kept away from bright light.
Rates
Internal users: Click here for rates.
External users: If you are an external client of Yale, please reach out to Jennifer Kelly for pricing and availability.
Contacts

Technical Director
PO Box 208035
333 Cedar St
New Haven, CT 06520-8035