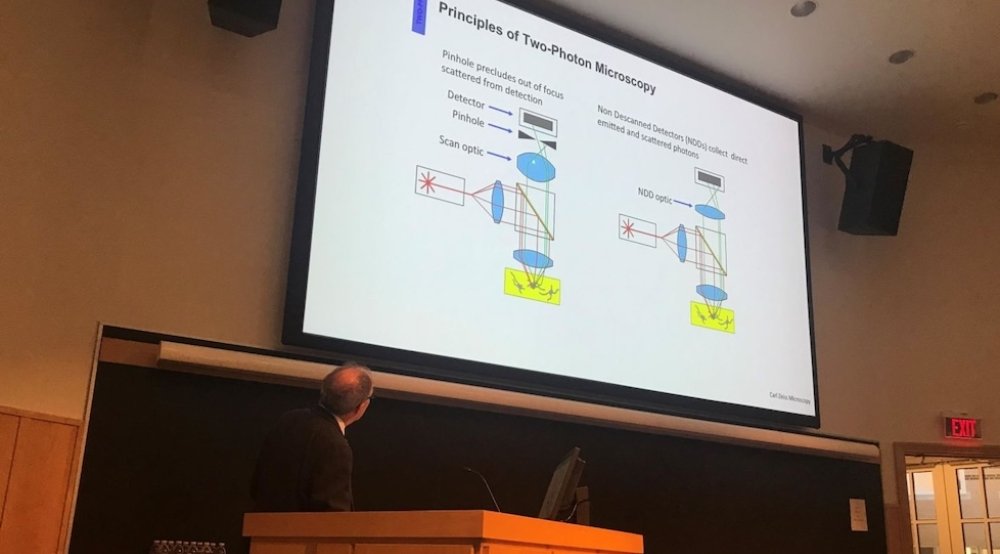
Confocal Microscopy at CCMI
Building: SHM IE-25
About the core
Confocal microscopy enables the visualization and imaging of fixed as well as living cells and tissues that contain fluorescent probes (fluorescent antibodies, fluorescent proteins, dyes, and substrates). This technique allows sharply defined optical sections to be collected, from which three-dimensional rendering and movies can be created.
Our facility, the Center for Cell and Molecular Imaging (CCMI), offers confocal microscopy, two-photon (multiphoton) microscopy, light sheet microscopy, swept-field microscopy, super-resolution imaging services, and image analysis to Yale and external investigators. It provides access to and training in the use of all microscopes, related equipment, and image workstations.
Equipment and services
- Leica SP5 confocal microscope
- Stellaris 8 DIVE multiphoton/confocal microscope
- Zeiss LSM 880 Airyscan confocal microscope
- Leica SP8 Gated STED 3X super-resolution microscope
- Bruker Opterra II swept-field microscope
- Bruker Luxendo light sheet microscope
- Bruker Vutara 352 super-resolution microscope
- Imaris image analysis workstations
In addition, 4 image workstations are available dedicated to image analysis. Software from Zeiss (ZEN black and blue), Leica (LAS X), Bitplane/Oxford (Imaris), and SVI (Huygens Deconvolution) are available to users for post-acquisition image analysis, which include modules for fluorescence deconvolution, 3D rendering, measurements of time-lapse data, and fluorescence co-localization.
Technical expertise is available to aid in experiment design, use of the equipment, and image analysis software.
Policies
General
- In order to gain access to the confocal facility and training, investigators must contact the director of the facility, Dr. Michael H. Nathanson, or Associate Director, Dr. Mateus Guerra.
- Instruments cannot be removed from the lab at any time except with staff authorization.
- Users of the facility should not store samples, solutions, or other materials within the facility unless authorized, and then only in designated areas. Any sample or solution left unlabeled will be discarded.
- Data stored in the Imaris workstations that is older than two months will be deleted without prior notice.
Instrument and specimen handling
- The staff is happy to provide users with instructions for the handling and cleaning of instruments and specimen handling in a safe manner.
- Spills need to be cleaned immediately and reported for further technical care. Individuals who do not report spills will not be able to use the facility again.
- Any slides with oil may not be used on microscopes with dry and water lenses.
- Any oil getting on a non-oil objective must be cleaned immediately with ethanol and reported to the facility manager.
- At the end of each session, users must clean the lenses used. Instructions for lens and specimen cleaning are posted near the microscopes.
Booking
- Microscope bookings are made through PPMS.
- Each lab is permitted to use each microscope a maximum of 4 hours per day and 8 hours per week during regular Monday-Friday work hours (8:00 am - 6:00 pm). There is no time limit for after-hours or weekend usage.
CCMI Core cancelation or no-show policy
- Users that reserve microscope time and do not show up will be billed for their reserved time as if it were used.
- Cancelations within 24 hours of scheduled reservation will be billed unless that time slot is subsequently booked by another user.
Training
- Training on confocal microscopes is provided free of charge to investigators with the permission of the Director.
- Training is performed by the staff of the facility only, unless with special authorization.
- For more information on training, please contact Dr. Mateus Guerra.
Computers
- Users are not allowed to download or install software on any of the computers without asking the staff. These computers are available exclusively for imaging samples, analysis and 3-4 dimensional reconstructions of pictures.
- Users should back up their files and delete their files from hard disks. Hard disks will be cleaned up on a regular basis without advance notice.
Publication acknowledgment
If research supported by this research core results in publication, please acknowledge this support by including the following in your publication(s): "We thank the Yale Center for Cellular & Molecular Imaging for usage of their _________ microscope(s).”
Available to Yale researchers & external researchers
Imaris 10.1: How to get started and new AI tools
Presented by the Center for Cellular & Molecular Imaging and the Yale Liver Center
Get started with Imaris 10.1
Imaris 10.1: How to get started and new AI toolsPresented by the Center for Cellular & Molecular Imaging and the Yale Liver Center
Learn moreRates
As of July 1, 2025
| Service | Yale faculty | Non-Yale user |
|---|---|---|
| Microscopes | $63/hour | $126/hr (for-profit users) $78.75/hr (non-profit users) |
| Training | Free | Free |
| Workstations | $5.25/hour | $7.35/hour for both for-profit and non-profit users |
CCMI Dean's Workshop, March 12, 2019
Confocal fluorescence microscopy is the most widely used method for advanced light microscopic imaging of biological specimens. However, several new technologies now permit fluorescence microscopic imaging that is either faster than standard (point-scanning) confocal microscopy, has better spatial resolution, or both. Rapid image collection techniques, which are ideal for examining live cells, tissues, and organisms, including swept field, multiphoton, and light-sheet microscopy were highlighted at the workshop. Super-resolution techniques, which can result in 2- to 10-fold improvement in spatial resolution relative to standard confocal microscopy, including gated STED, single-molecule localization, and Airyscan microscopy, were also discussed. The workshop illustrated the use of these new imaging techniques, each of which is now available in CCMI.
Downloadable forms
-
Leica STED Super Resolution Overview (10.24 MB)
-
STED sample preparation guide (5.49 MB)
-
STED sample prep chapter (281.82 KB)
Contacts
Sterling Hall of Medicine, room IE-25
333 Cedar Street
New Haven, CT 06510